Current News
Bryce Dixon Breitenstein
Health Physics Society (HPS) member Bryce Dixon Breitenstein died 27 November 2024. Dr. Breitenstein's obituary can be found on the HPS website In Memoriam page.
Occupational Internal Dosimetry Training Meeting at Georgia Tech
The Occupational Internal Dosimetry Training Meeting will be held 28 July–1 August 2025 at Georgia Institute of Technology (Georgia Tech) in Atlanta, Georgia. Registration is free and includes tours and an evening social mixer. The class will focus on three areas of internal dosimetry:
- ICRP 30, 68, and 130 biokinetics models and dosimetry schema as they relate to deriving secondary occupational dose limits (for example, ALIs and DACs).
- The design and implementation of occupational internal dose programs in USNRC and USDOE regulated facilities.
- The heart of occupational internal dosimetry: the methods and philosophy of evaluating occupational intakes of radioactive materials.
There will be example cases for the class to work through, but commercial software will not be provided—instead instruction will be provided on how to use spreadsheets (e.g., Excel, LibreOffice) to work example intake evaluations and occasionally use R (a freely available language for statistical computing) to tackle more challenging problems.
The course concludes with a workshop session where the class will have the opportunity to work example problems. No previous experience is required, and only freely available software will be used. The week will conclude with a workshop session where attendees will present aspects of their programs or share lessons learned. The workshops will be breakout sessions for Internal Dosimetry, In Vivo Monitoring and External Dosimetry.
The American Academy of Health Physics has awarded 40 continuing education credits for this training.
To register and find the schedule and more information, click here.
Instructors are Dr. Thomas LaBone and Dr. Charles "Gus" Potter.

Thomas LaBone
Thomas LaBone is an internal dosimetrist with over 40 years of operational experience in the radiopharmaceutical industry, US Department of Energy facilities, and the EEOICPA compensation program. He has been responsible for the design of internal dosimetry programs, the evaluation of intakes of fission products, tritium, uranium, and transuranics, and the mathematical modeling of biokinetic data. Tom completed a BS in chemistry, an MS in radiological science, an MS in industrial statistics, and a PhD in biostatistics. Tom lives in Aiken, South Carolina, with his wife Joan and four chihuahuas.

Gus Potter
Gus Potter is a senior scientist at Sandia National Laboratories, where he designed and implemented their internal dosimetry program. Over his 32-year career, he has published several papers on different aspects of internal dosimetry, including a seminal work for his PhD on the implementation of ICRP-68 models in intake and dose calculations. He has taught internal dosimetry at the University of New Mexico for over 30 years and continues to consult within the Sandia program and mentor internal dosimetrists. He participates in internal dosimetry standards working groups both with ISO and ANSI/HPS N13 working groups. Gus completed a BS in physics, an MS in radiological science, and a PhD in physics/radiological science. Gus lives in Albuquerque, New Mexico, with his wife Michelle.
70th HPS Annual Meeting: Meeting Sessions
Bill Hinchcliffe, 2025 Meeting Task Force Cochair
 As you might imagine, the 70th Health Physics Society Annual Meeting has felt the ripple effects of federal funding cutbacks, resulting in fewer abstract submissions than usual. But the Program Committee rose to the challenge and got creative! They've reimagined the schedule by starting most sessions at 9 am, extending talks to 20 minutes, and limiting the concurrent tracks to four instead of five—allowing us to build a strong program through Wednesday afternoon. While Thursday morning looked a little light at first, we're excited to share that it will now feature a robust lineup of Professional Enrichment Program sessions (PEPs)! This gives us a unique opportunity to spotlight these popular sessions in a dedicated time slot. Thursday morning's PEPs will be in addition to the Sunday lineup, but will be free to attend; however, advance registration is required—so don't forget to select your sessions when you register. We're excited about the program and can't wait to see you in Madison!
As you might imagine, the 70th Health Physics Society Annual Meeting has felt the ripple effects of federal funding cutbacks, resulting in fewer abstract submissions than usual. But the Program Committee rose to the challenge and got creative! They've reimagined the schedule by starting most sessions at 9 am, extending talks to 20 minutes, and limiting the concurrent tracks to four instead of five—allowing us to build a strong program through Wednesday afternoon. While Thursday morning looked a little light at first, we're excited to share that it will now feature a robust lineup of Professional Enrichment Program sessions (PEPs)! This gives us a unique opportunity to spotlight these popular sessions in a dedicated time slot. Thursday morning's PEPs will be in addition to the Sunday lineup, but will be free to attend; however, advance registration is required—so don't forget to select your sessions when you register. We're excited about the program and can't wait to see you in Madison!
From the President: IRPA Task Group on Education & Training – Five HPS Members Appointed
Liz Brackett, HPS President
 In early March, I received a message from Dr. Bernard le Guen, executive officer of the International Radiation Protection Association (IRPA), inviting Associate Societies to nominate members for a newly formed task group on education and training. With a deadline at the end of March, a call for volunteers was posted in Health Physics News.
In early March, I received a message from Dr. Bernard le Guen, executive officer of the International Radiation Protection Association (IRPA), inviting Associate Societies to nominate members for a newly formed task group on education and training. With a deadline at the end of March, a call for volunteers was posted in Health Physics News.
Despite the short turnaround, five Health Physics Society members expressed interest. All five nominations were submitted to the task group chair, and I'm pleased to report that last week I received notification that all five have been appointed.
The appointed members are:
- Emily Caffrey, PhD, CHP
- Margaret Chege, PhD
- Latha Vasudevan, PhD, CHP
- Jon Napier, PhD, CHP
- Dimitrios Kontogeorgakos, PhD, CHP
We look forward to seeing their contributions to this important initiative!
In Memoriam: Louie "Max" Scott
Health Physics Society (HPS) member Louie "Max" Scott died 11 March 2023. An In Memoriam piece can be found on the HPS website.
Donate to Health Physics Academic Education and Research Center
David Connolly, HPS Congressional Liaison and HPAERC Board Member
 Are you thinking about how to spend your tax refund? Here is an idea that will also benefit you on next year's tax return: MAKE A DONATION TO HPAERC!
Are you thinking about how to spend your tax refund? Here is an idea that will also benefit you on next year's tax return: MAKE A DONATION TO HPAERC!
As you are probably aware, for the past 10 years, there has been a significant decline in the number of academic programs for health physics at colleges and universities, resulting in a significant reduction in the number of graduates with a health physics degree entering the workforce each year. Clearly one of the biggest challenges facing the Health Physics Society (HPS) today is ensuring that the number of radiation professionals remains large enough to meet the overwhelming demand for their services throughout the United States.
In order to meet this critical shortage, the HPS Board of Directors founded the Health Physics Academic Education and Research Center (HPAERC), which is an independent 501(c)(3) nonprofit with its own Board of Directors. The goal of the HPAERC is to raise and disburse funds to support training in the profession of health physics to meet the critical shortage of health physicists in the country.
For this fiscal year, the HPS Board of Directors generously allocated funds for HPAERC to cover some of the administrative expenses related to its formation and operation, but More Donations Are Needed! In order for HPAERC to become a mature organization, a number of actions need to occur, including development of fundraising processes, solicitation and acknowledgement language for donors, website development, and income tracking, among others. In addition to donations, HPAERC is also looking for volunteers interested in helping the organization grow.
On behalf of the entire HPAERC Board of Directors and its advisors, we are asking you to make a contribution to the corporation so we can begin to make funds available for both individual student scholarships and support for health physics academic programs themselves.
Please make your checks to HPAERC and send them to HPS Headquarters, 950 Herndon Parkway, Suite 450, Herndon, VA 20170
For more information, contact HPAERC Board Chair Elizabeth Gillenwalters.
New HPS Website Launch Is Getting Closer!
Kendall Berry, MSPH, HPS Website Redevelopment & Deployment Task Force Chairperson
 We are getting closer!
We are getting closer!
I am thrilled to report that the new Health Physics Society website is getting closer to being launched! The Membership Committee has jumped in and quickly worked with the vendor developing our new member database, which has allowed us to get closer to launch.
Change is always exciting, but we know there will be bumps along the way. Once we launch, if you find any problems, please reach out to HPS Web Editor in Chief Barbara Hamrick, and the Web Ops team will work to find a solution.
I hope you are as excited as we all are!
Occupational Internal Dosimetry Training Meeting at Georgia Tech
The Occupational Internal Dosimetry Training Meeting will be held 28 July–1 August 2025 at Georgia Institute of Technology (Georgia Tech) in Atlanta, Georgia. Registration is free and includes tours and an evening social mixer. The class will focus on three areas of internal dosimetry:
- ICRP 30, 68, and 130 biokinetics models and dosimetry schema as they relate to deriving secondary occupational dose limits (for example, ALIs and DACs).
- The design and implementation of occupational internal dose programs in USNRC and USDOE regulated facilities.
- The heart of occupational internal dosimetry: the methods and philosophy of evaluating occupational intakes of radioactive materials.
There will be example cases for the class to work through, but commercial software will not be provided—instead instruction will be provided on how to use spreadsheets (e.g., Excel, LibreOffice) to work example intake evaluations and occasionally use R (a freely available language for statistical computing) to tackle more challenging problems.
The course concludes with a workshop session where the class will have the opportunity to work example problems. No previous experience is required, and only freely available software will be used. The week will conclude with a workshop session where attendees will present aspects of their programs or share lessons learned. The workshops will be breakout sessions for Internal Dosimetry, In Vivo Monitoring and External Dosimetry.
The American Academy of Health Physics has awarded 40 continuing education credits for this training.
To register and find the schedule and more information, click here.
Instructors are Dr. Thomas LaBone and Dr. Charles "Gus" Potter.

Thomas LaBone
Thomas LaBone is an internal dosimetrist with over 40 years of operational experience in the radiopharmaceutical industry, US Department of Energy facilities, and the EEOICPA compensation program. He has been responsible for the design of internal dosimetry programs, the evaluation of intakes of fission products, tritium, uranium, and transuranics, and the mathematical modeling of biokinetic data. Tom completed a BS in chemistry, an MS in radiological science, an MS in industrial statistics, and a PhD in biostatistics. Tom lives in Aiken, South Carolina, with his wife Joan and four chihuahuas.

Gus Potter
Gus Potter is a senior scientist at Sandia National Laboratories, where he designed and implemented their internal dosimetry program. Over his 32-year career, he has published several papers on different aspects of internal dosimetry, including a seminal work for his PhD on the implementation of ICRP-68 models in intake and dose calculations. He has taught internal dosimetry at the University of New Mexico for over 30 years and continues to consult within the Sandia program and mentor internal dosimetrists. He participates in internal dosimetry standards working groups both with ISO and ANSI/HPS N13 working groups. Gus completed a BS in physics, an MS in radiological science, and a PhD in physics/radiological science. Gus lives in Albuquerque, New Mexico, with his wife Michelle.
70th HPS Annual Meeting: Meeting Sessions
Bill Hinchcliffe, 2025 Meeting Task Force Cochair
 As you might imagine, the 70th Health Physics Society Annual Meeting has felt the ripple effects of federal funding cutbacks, resulting in fewer abstract submissions than usual. But the Program Committee rose to the challenge and got creative! They've reimagined the schedule by starting most sessions at 9 am, extending talks to 20 minutes, and limiting the concurrent tracks to four instead of five—allowing us to build a strong program through Wednesday afternoon. While Thursday morning looked a little light at first, we're excited to share that it will now feature a robust lineup of Professional Enrichment Program sessions (PEPs)! This gives us a unique opportunity to spotlight these popular sessions in a dedicated time slot. Thursday morning's PEPs will be in addition to the Sunday lineup, but will be free to attend; however, advance registration is required—so don't forget to select your sessions when you register. We're excited about the program and can't wait to see you in Madison!
As you might imagine, the 70th Health Physics Society Annual Meeting has felt the ripple effects of federal funding cutbacks, resulting in fewer abstract submissions than usual. But the Program Committee rose to the challenge and got creative! They've reimagined the schedule by starting most sessions at 9 am, extending talks to 20 minutes, and limiting the concurrent tracks to four instead of five—allowing us to build a strong program through Wednesday afternoon. While Thursday morning looked a little light at first, we're excited to share that it will now feature a robust lineup of Professional Enrichment Program sessions (PEPs)! This gives us a unique opportunity to spotlight these popular sessions in a dedicated time slot. Thursday morning's PEPs will be in addition to the Sunday lineup, but will be free to attend; however, advance registration is required—so don't forget to select your sessions when you register. We're excited about the program and can't wait to see you in Madison!
From the President: IRPA Task Group on Education & Training – Five HPS Members Appointed
Liz Brackett, HPS President
 In early March, I received a message from Dr. Bernard le Guen, executive officer of the International Radiation Protection Association (IRPA), inviting Associate Societies to nominate members for a newly formed task group on education and training. With a deadline at the end of March, a call for volunteers was posted in Health Physics News.
In early March, I received a message from Dr. Bernard le Guen, executive officer of the International Radiation Protection Association (IRPA), inviting Associate Societies to nominate members for a newly formed task group on education and training. With a deadline at the end of March, a call for volunteers was posted in Health Physics News.
Despite the short turnaround, five Health Physics Society members expressed interest. All five nominations were submitted to the task group chair, and I'm pleased to report that last week I received notification that all five have been appointed.
The appointed members are:
- Emily Caffrey, PhD, CHP
- Margaret Chege, PhD
- Latha Vasudevan, PhD, CHP
- Jon Napier, PhD, CHP
- Dimitrios Kontogeorgakos, PhD, CHP
We look forward to seeing their contributions to this important initiative!
In Memoriam: Louie "Max" Scott
Health Physics Society (HPS) member Louie "Max" Scott died 11 March 2023. An In Memoriam piece can be found on the HPS website.
Donate to Health Physics Academic Education and Research Center
David Connolly, HPS Congressional Liaison and HPAERC Board Member
 Are you thinking about how to spend your tax refund? Here is an idea that will also benefit you on next year's tax return: MAKE A DONATION TO HPAERC!
Are you thinking about how to spend your tax refund? Here is an idea that will also benefit you on next year's tax return: MAKE A DONATION TO HPAERC!
As you are probably aware, for the past 10 years, there has been a significant decline in the number of academic programs for health physics at colleges and universities, resulting in a significant reduction in the number of graduates with a health physics degree entering the workforce each year. Clearly one of the biggest challenges facing the Health Physics Society (HPS) today is ensuring that the number of radiation professionals remains large enough to meet the overwhelming demand for their services throughout the United States.
In order to meet this critical shortage, the HPS Board of Directors founded the Health Physics Academic Education and Research Center (HPAERC), which is an independent 501(c)(3) nonprofit with its own Board of Directors. The goal of the HPAERC is to raise and disburse funds to support training in the profession of health physics to meet the critical shortage of health physicists in the country.
For this fiscal year, the HPS Board of Directors generously allocated funds for HPAERC to cover some of the administrative expenses related to its formation and operation, but More Donations Are Needed! In order for HPAERC to become a mature organization, a number of actions need to occur, including development of fundraising processes, solicitation and acknowledgement language for donors, website development, and income tracking, among others. In addition to donations, HPAERC is also looking for volunteers interested in helping the organization grow.
On behalf of the entire HPAERC Board of Directors and its advisors, we are asking you to make a contribution to the corporation so we can begin to make funds available for both individual student scholarships and support for health physics academic programs themselves.
Please make your checks to HPAERC and send them to HPS Headquarters, 950 Herndon Parkway, Suite 450, Herndon, VA 20170
For more information, contact HPAERC Board Chair Elizabeth Gillenwalters.
April Course Listings
The April course offerings have been posted on the Course Listings page of the HPS website. Information on the following courses is available:
Laser Safety Officer (LSO) Training—Kentek Corporation
Certification Review Course Part II and Self Study Course Part II—Bevelacqua Resources
Gamma Spectroscopy—ORAU's Professional Training Programs
MARSSIM for Managers Online Training Course—ORAU's Professional Training Programs
Facility Decommissioning Training Course—Argonne National Laboratory (ANL)
Social Program During 70th HPS Annual Meeting in Madison
Mike Lewandowski, FHPS, CHP, Local Arrangements Committee


Wisconsin State Capitol from Monona Terrace
Photo by Sharon Vanorney, courtesy of Destination Madison

Sample a variety of local beers
Photo by Sharon Vanorney, courtesy of Destination Madison
We're just three months away from the 70th Health Physics Society Annual Meeting, being held 13–17 July 2025 in Madison, Wisconsin. It's time to book a hotel and prepare to visit Madison. In addition to an excellent technical program, you'll be able to enjoy an exceptional sampling of what the city has to offer. The Local Arrangements Committee (LAC) has developed a comprehensive program of social tours and events that are sure to please.
This month we'll highlight two components of the social program: social tours and the microbrewery tour. Start your week in Madison by getting oriented to the city and state. Highlights of Monday's social program include a guided tour of the Wisconsin State Capitol (located a few blocks from the meeting hotels and Monona Terrace Convention Center). Follow the Capitol tour with a guided tour down State Street, which is filled with shopping and dining options that are sure to keep you busy for the rest of the week. After lunch there is a Cultural Landscape Tour to learn more about this part of Madison and the people who have occupied this area for more than 12,000 years.
Tuesday's event is a day-long tour of the nearby New Glarus community. This historic Swiss village transports the visitor to a European environment uniquely blended with Wisconsin culture. Be sure to enjoy the Swiss Historical Village and Museum, Bailey's Run Vineyard, and the New Glarus Brewing Company. A bus will leave from and return to the Monona Terrace Convention Center.
Wednesday's events include a sampling of the famous Dane County Farmers Market on the Capitol square. This is a perfect way to start your day with fresh fruits, vegetables, and baked goods. Don't miss a self-guided tour of the Olbrich Botanical Gardens and Bolz Conservatory. The variety of colors and textures is a feast for the eye.
No visit to Wisconsin is complete without paying homage to our beer-brewing history. German immigrants brought their brewing expertise with them and established Wisconsin as a center of the beer industry. After all, our Major League Baseball team is called the Brewers. Join us on Tuesday evening for "A Curie for Your Ales" brewery tour. We'll walk to three different microbreweries in downtown Madison so you can sample their wares and choose your favorite. Each registered tour attendee will receive a commemorative pint glass in addition to a free drink at each stop.
You can find more details about these events as well as details about the entire meeting on the Madison meeting microsite. Check back often as the LAC, Program Committee, and Headquarters staff will be adding information about the meeting to this site up until the meeting.
If you have questions about the meeting or Madison, feel free to contact LAC Cochairs Jessica Joyce and Jason Rusch.
Health Physics Society Annual Meeting – July 2025 (Planning in Progress)

This article was written by ChatGPT with some input from me. Its style is a bit more exuberant than mine, but I am excited about the plenary session and would like to thank Drs. Emily Caffrey and Shaheen Dewji for their efforts in putting it together.
--Liz Brackett

HPS President Liz Brackett
Picture generated with Apple's Image Playground app with a request to make the submitted profile headshot professional
We are thrilled to share that we are currently in the planning stages for our 70th HPS Annual Meeting, set to take place 13–17 July 2025 in Madison, Wisconsin. While we are still finalizing details, we wanted to give you a sneak peek at what promises to be an exciting and highly informative event.
Plenary Session: The Role of AI in Radiation Protection
One of the key highlights of this year's conference will be our plenary session, "The Use of Artificial Intelligence in Radiation Protection." AI is making significant strides in many fields, and radiation protection is no exception. During this session, we will delve into the transformative potential of AI for improving radiation safety, from enhanced monitoring and real-time risk assessment to more efficient radiation treatment and protection protocols.
Our expert speakers will discuss how AI can help us address ongoing challenges and create new opportunities for safety and efficiency across a variety of industries, including medical applications, dose reconstruction, nuclear energy, and environmental protection. This will be a unique chance to learn about the intersection of cutting-edge technology and radiation safety.
Premeeting Teaser Webinar: Introduction to AI
To build anticipation for the plenary session and ensure our members are prepared for this exciting topic, we are hosting a teaser webinar in the coming months. This introductory session will provide a comprehensive overview of AI, its applications in radiation protection, and the broader implications for our profession. Whether you're new to AI or looking to expand your knowledge, this webinar will serve as a valuable primer on the topic ahead of the July meeting.
We encourage all members to register for the teaser webinar and stay tuned for further details about the annual meeting. Whether you're a radiation safety professional, researcher, or industry leader, this meeting will offer valuable insights into the future of radiation protection and the transformative role AI will play in shaping it.
Mark Your Calendars
- Teaser Webinar on AI: Date TBD (stay tuned for updates)
- Annual Meeting: 13–17 July, Monona Terrace Convention Center, Madison, WisconsinI
We look forward to engaging with our members on these exciting developments and sharing the latest advancements in radiation protection. Your participation is what makes this event a hub of innovation, collaboration, and knowledge-sharing. Together, we can continue to ensure a safer and more innovative future for our field.
Stay connected through Health Physics News and the meeting website for more details and registration information. We hope to see you in July!
George Tabatadze Project on Radon in Tbilisi Funded

George Tabatadze
Health Physics Society member George Tabatadze's three-year project, "Protecting Public Health through Comprehensive Radon Monitoring and Dosimetry in Urban Tbilisi," has been funded by the Shota Rustaveli National Science Foundation of Georgia (SRNSFG) and will be conducted at Georgian Technical University. This initiative aims to enhance public health protection by advancing radon monitoring and dosimetry research in Georgia. George says thank you to everyone who has supported this work, and he looks forward to the impact this research will have.
Sara Dumit, Philip Egidi, and Angela Leek Elected to NCRP

At the 2025 NCRP Annual Meeting, left to right, Philip Egidi, Angela Leek, and Sara Dumit
Photo courtesy of Sara Dumit
Health Physics Society members Sara Dumit, PhD, Philip Egidi, and Angela Leek, PhD, CHP, were recently elected as members of the National Council on Radiation Protection and Measurements (NCRP). Members are selected on the basis of their scientific expertise and serve six-year terms.
The NCRP was chartered by the US Congress in 1964 (Public Law 88-376). Its mission is to develop and disseminate information, guidance, and recommendations on radiation protection and measurements, reflecting the consensus of leading scientific expertise.
The NCRP plays a crucial role in fostering collaboration among organizations engaged in the scientific and related aspects of radiation protection and measurements.
San Diego Chapter March Meeting
Bridget Smith, Chapter Secretary
 The San Diego Chapter of the Health Physics Society met on 20 March 2025, hosted by the Radiological Health Program of the San Diego Department of Environmental Health and Quality. Host Ron Yonemitsu, San Diego County senior health physicist, shared a new tool that the county has acquired to analyze and identify unknown radioactive sources. Dr. Daniel Scanderbeg, associate director of the Division of Medical Physics at UCSD, gave the excellent presentation "Innovations in Radiation Medicine and Infraguard." You can view Scanderbeg's talk on the San Diego Chapter YouTube Channel.
The San Diego Chapter of the Health Physics Society met on 20 March 2025, hosted by the Radiological Health Program of the San Diego Department of Environmental Health and Quality. Host Ron Yonemitsu, San Diego County senior health physicist, shared a new tool that the county has acquired to analyze and identify unknown radioactive sources. Dr. Daniel Scanderbeg, associate director of the Division of Medical Physics at UCSD, gave the excellent presentation "Innovations in Radiation Medicine and Infraguard." You can view Scanderbeg's talk on the San Diego Chapter YouTube Channel.
We are looking forward to hosting a possible social event for members, a tour of San Onofre Nuclear Power Plant, and more online and virtual meetings in 2025.

Dr. Daniel Scanderbeg discussing innovations in radiation medicine
Photo courtesy of Bridget Smith

Chapter President Brianna Tuma-Marcella conducting chapter business
Photo courtesy of Rene Michel
American Nuclear Society Chornobyl Presentation Summary
Ken Gavlik, Decommissioning Section President
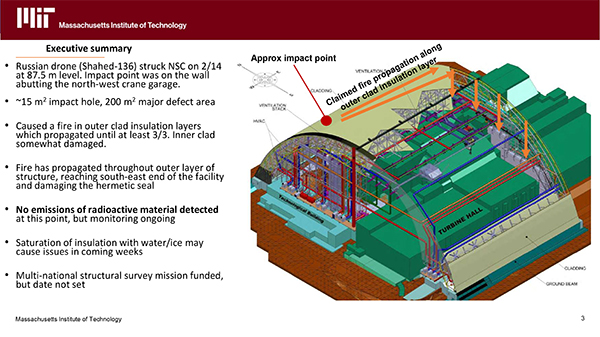 The Health Physics Society's Decommissioning Section current president and president-elect were pleased to attend the American Nuclear Society's very informative briefing on 19 March 2025 by Dr. Jake Hecia on a drone strike on the Ukraine's Chornobyl New Safe Confinement (NSC) and resulting fire and structural damage.
The Health Physics Society's Decommissioning Section current president and president-elect were pleased to attend the American Nuclear Society's very informative briefing on 19 March 2025 by Dr. Jake Hecia on a drone strike on the Ukraine's Chornobyl New Safe Confinement (NSC) and resulting fire and structural damage.
Hecia described how, on 14 February 2025, a Russian Shahed-136 drone struck the NSC at the 87.5-meter level, hitting the wall near the northwest crane garage. The impact created a 15 m² hole and a 200 m² major defect area, igniting a fire in the outer clad insulation layers (likely fueled by gasoline from the drone). The fire spread through the EPDM (synthetic rubber) layer and possibly the mastic, despite the EPDM's supposed fire resistance, reaching the southeast end of the facility by early March and damaging the hermetic seal, including a 2 km polyethylene sealing membrane. Firefighting efforts involved punching holes in the Kalzip outer layer and pumping in water, which extinguished open flames by March 6 but led to smoldering and water/ice buildup in the insulation, posing future risks. Thermal drones aided firefighting by identifying hot spots. No increased radiation levels or radioactive aerosol emissions have been detected, though monitoring continues. The inner clad layer sustained minor damage, but the outer cladding is extensively compromised, no longer meeting leak specifications, which could allow dust release. A multi-national structural survey is funded but unscheduled. Short-term, there's no immediate structural danger, but long-term, the NSC may require re-cladding (challenging due to high radiation levels) and replacement of flooded monitoring equipment. The situation was unexpected, and no clear remediation plan exists yet.
Decommissioning Section News
Ken Gavlik, Section President
The Health Physics Society (HPS) Decommissioning Section is proud to announce continuation of its webinar series with more information about the San Onofre Nuclear Generating Station (SONGS).
In August 2023, the Decommissioning Section hosted the webinar presentation "Overview of San Onofre Nuclear Generating Station Decommissioning."
On 24 April 2025, 2 pm PDT (5 pm EDT), the Decommissioning Section will continue the series with participation from San Onofre personnel Bill Barley and Emery Grohregin with the presentation "SONGS Decommissioning and Partial Site Release." See below for registration details.
Speaker Biographies:
- William Barley is currently assisting Southern California Edison with the SONGS decommissioning end state determination and License Termination Plan development. Prior to SONGS, he was the site closure manager of Humboldt Bay Power Plant in Northern California, responsible for the License Termination Plan, Final Status Survey Program, US Nuclear Regulatory Commission (NRC) interface, radiation protection, training, and on-site laboratory support to the project.
Barley has more than 50 years of experience in nuclear power, with 30 years of that experience being nuclear decommissioning experience in NRC, US Department of Energy, and United Kingdom facilities. He has a BS degree in chemical engineering from Penn State University and is a certified health physicist by the American Board of Health Physics (ABHP). He is a past licensed senior reactor operator engineer, NRC Inspector at TMI-2, and has served on the ABHP Part I and Part II certification panels.
- Emery Grohregin is an oversight specialist for the final status and license termination project of the SONGS in Southern California. He has been involved in previous nuclear decommissioning projects including Humboldt Bay, Rancho Seco, Crystal River, and Vermont Yankee.
Grohregin has more than 30 years of experience in the nuclear field, including reactor power operations and maintenance and commercial laboratory experience. He is certified in comprehensive health physics by the ABHP.
Please click here to sign up for the presentation. The deadline to register is 20 April. A link will be sent to registered attendees by 21 April.
In addition, the Decommissioning Section is also solicitating volunteers to conduct additional decommissioning-centric webinar presentations. Please email your interest, topic, and summary to Ken Gavlik, Kevin Banks, or Phil Rutherford for consideration.
Health Physics Editor's Note: Deck Reading
Brant Ulsh, CHP, PhD, Health Physics Editor in Chief

It's time to head out to the deck or patio and enjoy some spring sunshine and warm weather! Don't forget to take your tablet or laptop with you so you can soak up some knowledge along with the rays. The May issue of the Health Physics Journal is crammed full of the latest radiation protection research:
- "Evaluation of a Commercially Available Radiochromic Film for Use as a Complementary Dosimeter for Rapid In-Field Low Photon Equivalent Radiation Dose (≤50 mSv) Monitoring" by Nicky Nivi, Helen Moise, and Ana Pejovic-Milic
- "Study on the Effect of Adipose Tissue on Neutron Dose Evaluation for the Human Body Using Voxel Phantoms with Different Weight" by Xu Xu, Yong Yuan, and Xiao-Min Zhang
- "A Study of Radium Content and Radon Exhalation Rates in Soil Samples from Abi-Adi Town, Ethiopia, Using LR-115 Type-II" by Nigus Alene Assefa and Yisak Arbese
- "Three Developments Related to ANSI/HPS Standard 13.56" by William Evans
- "Gafchromic Films as a Complementary In-Field Dosimetric Tool to Monitor Low Photon Radiation Doses (≤50 mSv)" by Nicky Nivi, Helen Moise, and Ana Pejovic-Milic
- "Characterization of Radionuclide Resuspension via Aeolian Processes at a Uranium Mill Tailings Site" by Vanessa Adriatico and Camille Palmer
- "Implementation of Stochastic Gradient Descent in an Automated Glow Peak Identification Software for Multiple Thermoluminescent Dosimeter Types" by Jordan D. Noey, Colin J. Stewart, Wenjin Yu, and Kimberlee J. Kearfott
- "Peripheral Nerve Stimulation Thresholds Based on Waveform Shape and Implications for Guideline Limits" by Gregory B. Gajda
- "Systematical Theoretical Study of the Nonpoint Source Effects in Nuclear Medicine Shielding Calculation" by Tianlian Gu
- "Pre-Declaration Fetal Dose Assignment and Predictive Full-Term Fetal Dose at Medical Facilities" by James Kyle Underwood
The birds singing, a cold glass of iced tea, flowers blooming everywhere, and the May issue of Health Physics! What could be better?
ICRP Report Available for Consultation
Wayne Glines, Health Physics News Contributing Editor
 International Commission on Radiological Protection (ICRP) Task Group 91 draft report "Scientific Evidence Relevant to the Assessment of Solid Cancer Radiation Risk at Low Dose and Low Dose Rate" is now available for public consultation. Individuals and organizations are welcome to provide comments before the deadline on 13 June 2025. This draft report and a link for submitting comments may be found on the ICRP Consultation Page. A digital workshop seeking feedback on the report will be scheduled during the consultation period. Information and registration for this workshop will be provided at a later date.
International Commission on Radiological Protection (ICRP) Task Group 91 draft report "Scientific Evidence Relevant to the Assessment of Solid Cancer Radiation Risk at Low Dose and Low Dose Rate" is now available for public consultation. Individuals and organizations are welcome to provide comments before the deadline on 13 June 2025. This draft report and a link for submitting comments may be found on the ICRP Consultation Page. A digital workshop seeking feedback on the report will be scheduled during the consultation period. Information and registration for this workshop will be provided at a later date.
HPS Leaders Attend NCRP Meeting

Current, future, and past presidents of the Health Physics Society attended the 2025 National Council on Radiation Protection and Measurements Annual Meeting in March. Left to right: Ruth McBurney, Eric Goldin, Barbara Hamrick, Armin Ansari, President Liz Brackett, President-elect Mike Lewandowski, Kathy Prior, and Ken Kase
Photo courtesy of Jim Willison
Join Our Certified Health Physicist Exam Study Group!
HPS Society Support Committee
 Preparing for the certified health physicist exam? We've got you covered! Our study group is designed to help you dive deep into the exam topics without the stress. Starting 2 April 2025, we'll meet every Wednesday, 19:00-21:00 EST, to focus on key areas like Measurements & Instrumentation, Standards, Hazard Analysis, and much more.
Preparing for the certified health physicist exam? We've got you covered! Our study group is designed to help you dive deep into the exam topics without the stress. Starting 2 April 2025, we'll meet every Wednesday, 19:00-21:00 EST, to focus on key areas like Measurements & Instrumentation, Standards, Hazard Analysis, and much more.
These sessions are designed with YOU in mind—perfect for busy professionals balancing work and study. Expect interactive, discussion-based activities that let you reinforce what you've learned, without adding extra pressure. We'll focus on real-world applications, practical exercises, and group discussions to help you build confidence and prepare efficiently.
What you can expect:
- Weekly 2-hour sessions (2 April to 4 June, 19:00-21:00 EST)
- Engaging, relaxed, and collaborative activities
- Focus on real-world scenarios and exam-relevant content
- Last-minute exam prep and Q&A before the big day!
Session Breakdown:
- 2 April: Instruments & Measurements – Explore radiation measurement tools, calibration, and quality-control methods.
- 9 April: Sampling & Data Analysis – Dive into statistical data analysis, air sampling devices, and internal dose calculations.
- 16 April: Standards, Reporting, & Quality Control – Understand regulations, compliance, TEDE calculations, and report analysis.
- 23 April: Hazards & Engineering Controls – Learn about hazard identification, engineered controls, shielding, and PPE selection.
- 30 April: Hazards & Regulation – Review shielding, compliance, fault tree analysis, and calibration protocols.
- 7 May: Controls & Procedures – Discuss emergency response plans, record-keeping, and best practices in operations.
- 14 May: Operations & Emergency Protocols – Focus on SOPs, contamination control, and scenario-based procedure development.
- 21 May: Programmatic Design & Compliance – Dive into ALARA principles, radiation exposure risk, and effective communication strategies.
- 28 May: Training Programs & Fundamentals Review – Review core concepts like radiation effects, training modules, and simplification strategies.
- 4 June: Final Review & Exam Prep – Targeted review of key topics, practice problems, and exam prep tips.
Register and Sign Up:
Don't miss this opportunity to get exam-ready with the support of your peers. Email SSC@HPS.org to register today and secure your spot as a student or a mentor!
Central Rocky Mountain Chapter March Meeting
Deirdre Elder, Chapter President

At the Central Rocky Mountain Chapter meeting, left to right, Matthew Gift, Deirdre Elder, Katherine Liberman, Cheri Douglas, and Andrew Halloran
Photo courtesy of James DeWolfe
 The Central Rocky Mountain Chapter of the Health Physics Society met on 12 March 2025 to discuss Human Use Research with Radiation and Radioactive Materials. Most of us began with a light dinner and social hour, though the meeting was also available virtually. Matthew Gift, CHP, and Katherine Liberman from the Colorado Department of Public Health and Environment shared a regulatory perspective. Andrew Halloran, CHP, described the role of health physicists in human research using ionizing radiation and the approach taken at the University of Colorado Anschutz. Deirdre Elder, CHP, explained the types of studies performed at the academic medical center, University of Colorado Hospital and the process for approval for the UCHealth system. Cheri Douglas, CHP shared the perspective of a contract radiation safety officer for limited scope radioactive materials licensed facilities. The presentations were followed by a lively panel discussion and all came away with an improved understanding of the research being performed using ionizing radiation and the relevant regulations and safety procedures.
The Central Rocky Mountain Chapter of the Health Physics Society met on 12 March 2025 to discuss Human Use Research with Radiation and Radioactive Materials. Most of us began with a light dinner and social hour, though the meeting was also available virtually. Matthew Gift, CHP, and Katherine Liberman from the Colorado Department of Public Health and Environment shared a regulatory perspective. Andrew Halloran, CHP, described the role of health physicists in human research using ionizing radiation and the approach taken at the University of Colorado Anschutz. Deirdre Elder, CHP, explained the types of studies performed at the academic medical center, University of Colorado Hospital and the process for approval for the UCHealth system. Cheri Douglas, CHP shared the perspective of a contract radiation safety officer for limited scope radioactive materials licensed facilities. The presentations were followed by a lively panel discussion and all came away with an improved understanding of the research being performed using ionizing radiation and the relevant regulations and safety procedures.




